Technology
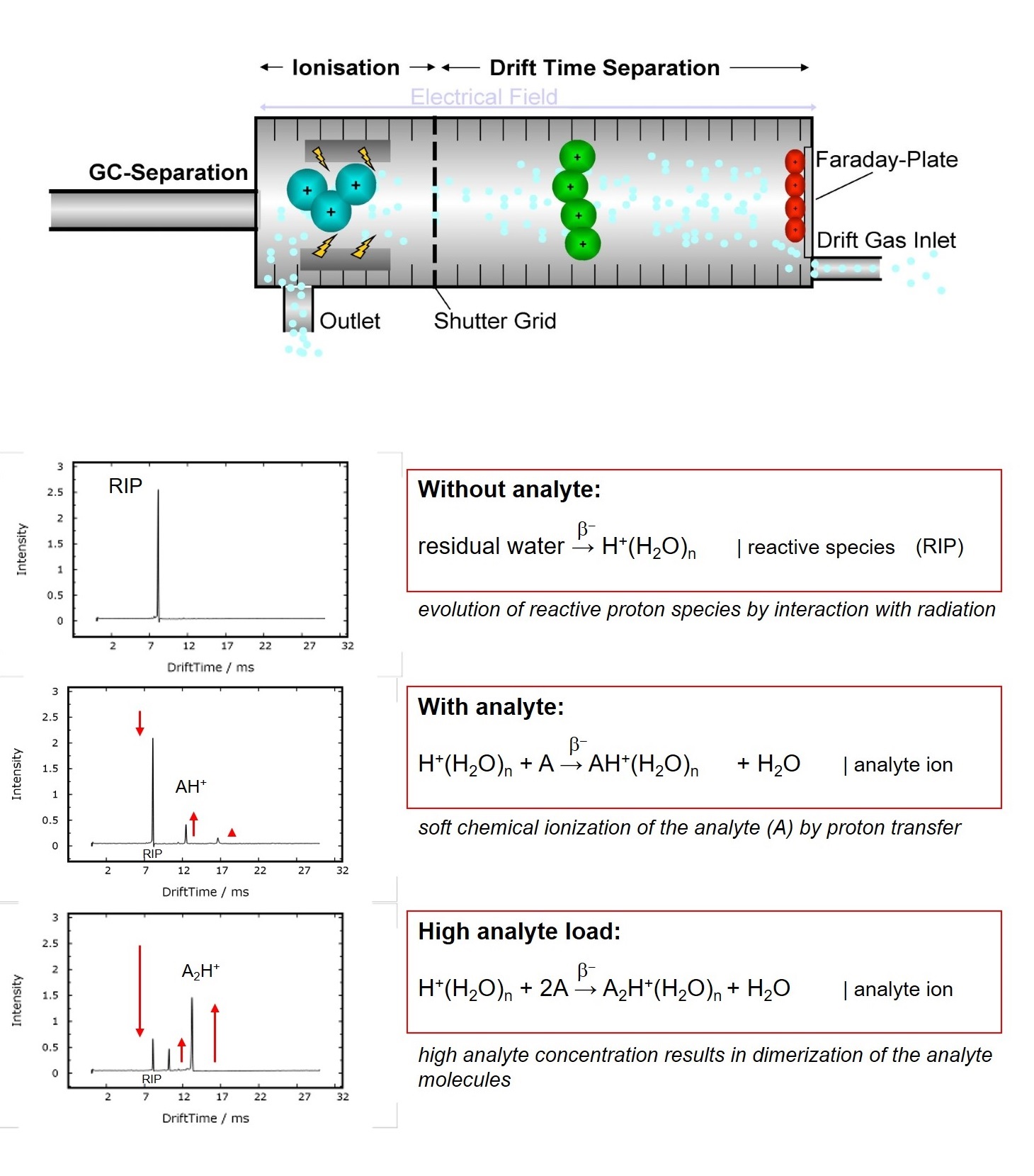
Ion Mobility Spectrometry (IMS) is an analytical technology to separately detect gaseous compounds in a mixture of analytes. The separation is based on the specific drift times, that ionized compounds need to pass a fixed distance (drift tube) in a defined electric field
Compared to other techniques e.g. TOF-MS, ions travel at atmospheric pressure versus a flow of inert drift gas. The drift time of each substance is determined by its ion‘s mass and geometric structure, as slowing collisions with the drift gas molecules are more frequent for sterically demanding structures. Therefore IMS can even differentiate isobaric molecules.
For detection, the resulting ion current is measured by an electrometer as a function of time. Atmospheric Ionization of molecules can be obtained by several techniques. G.A.S. uses photoionization with a 10.6eV UV-lamp or soft chemical-ionization initiated by a low-radiation tritium (H3) source (below excemption limits of EURATOM). While the first directly produces positive ions, the latter generates rectand ions with the gas atmosphere by a cascade of reactions following the collision of a fast electron emitted from the β-radiator H3[1]. The so-called Reaction Ion Peak (RIP) representing the total of all ions available is formed. In nitrogen and air, resp., the reactand ions can be descibed as H+(H2O)n and O2-(H2O)n. Chemical ionization of analytes by reactand ions then result in the formation of specific analyte ions, when the affinity of the analyte towards the reactand ion is higher when compared to water. The proton affinity of water is 691kJ/mol, so all molecules with a higher proton affinity will be ionized by proton transfer, which is typically given for all heteroatom-organic compounds.
G.A.S. Gesellschaft für analytische Sensorsysteme mbH
BioMedizinZentrumDortmund
Otto-Hahn-Str. 15
44227 Dortmund
Germany
Phone : +49 231 9742 6550
Fax : +49 231 9742 6555
E-Mail : info@GAS-Dortmund.de
IMS Working Principle
Ion Mobility Spectrometry (IMS) is an analytical technology to separately
detect gaseous compounds in a mixture of analytes. The separation is based on
the specific drift times, that ionized compounds need to pass a fixed
distance (drift tube) in a defined electric field.
Compared to other techniques e.g. TOF-MS, ions travel at atmospheric
pressure versus a flow of inert drift gas. The drift time of each
substance is determined by its ion‘s mass and geometric structure, as slowing
collisions with the drift gas molecules are more frequent for sterically
demanding structures. Therefore IMS can even differentiate isobaric molecules.
For detection, the resulting ion current is measured by an electrometer as a
function of time. Atmospheric Ionization of molecules can be obtained by several
techniques. G.A.S. uses soft chemical-ionization initiated by a low-radiation tritium (H3) source (below
excemption limits of IAEA resp. EURATOM).
In a first step rectand ions are generated by a cascade of reactions following the collision of a fast electron emitted from the β-radiator with the drift gas atmosphere[1]. As a consequence the so-called Reaction Ion Peak (RIP) representing the total of all ions available is formed. In nitrogen and air, resp., the reactand ions can be descibed as H+(H2O)n and O2-(H2O)n. Chemical ionization of analytes by reactand ions then result in the formation of specific analyte ions, when the affinity of the analyte towards the reactand ion is higher when compared to water. The proton affinity of water is 691kJ/mol, so all molecules with a higher proton affinity will be ionized by proton transfer, which is typically given for all heteroatom-organic compounds.
[1] Eiceman, G. and Karpas, Z., Ion Mobility Spectrometry, ISBN 0-88493-2247-2
Gas Chromatography (GC) coupled to Ion Mobility Spectrometry (IMS)
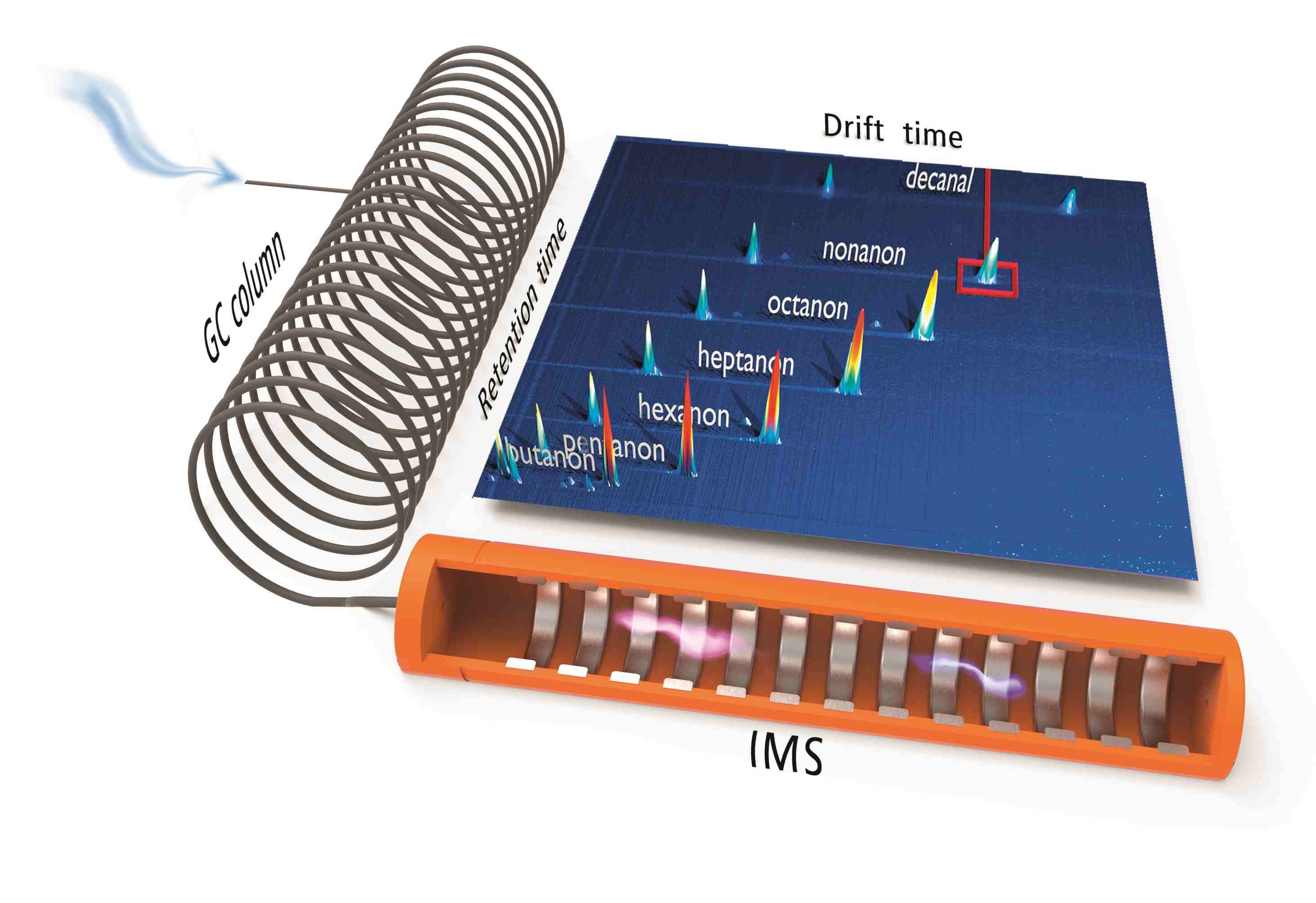
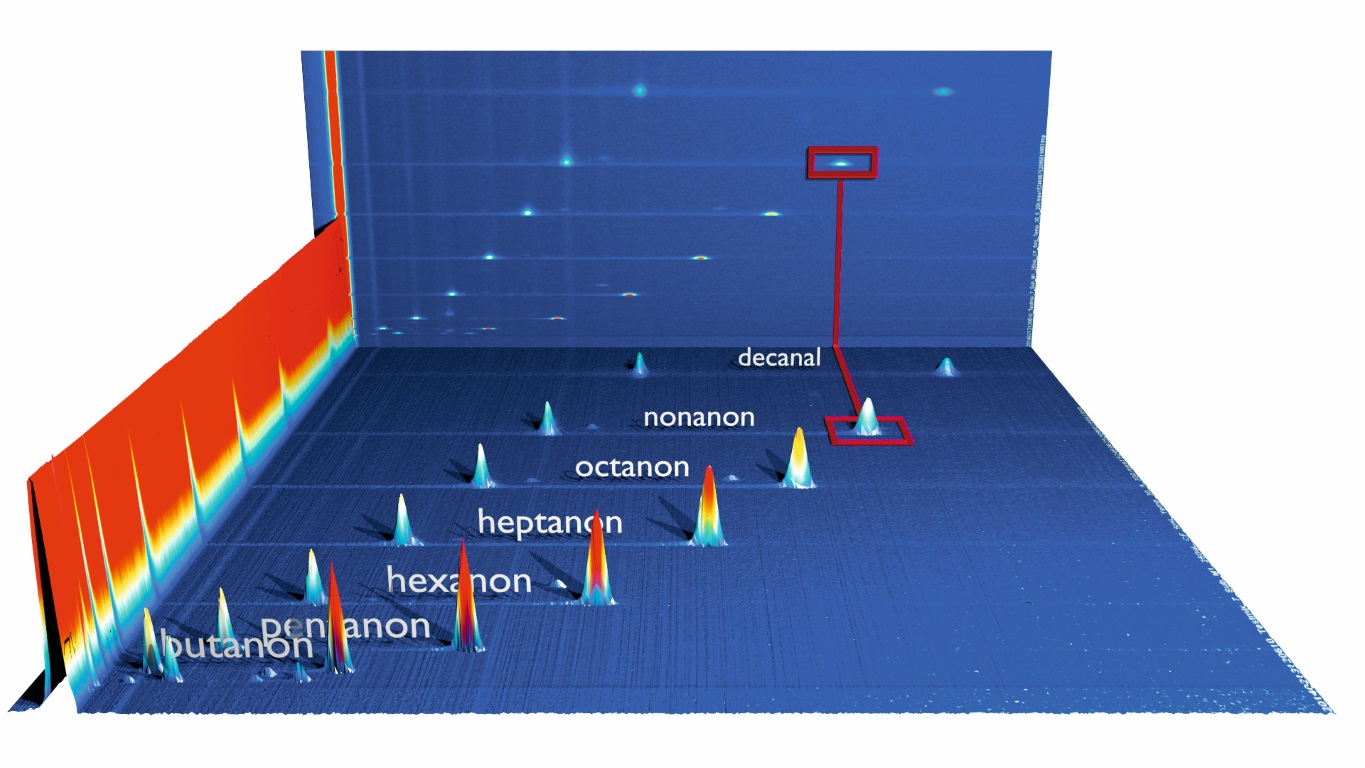
Complex analyte mixtures like for example food, flavours, natural gas and environmental applications often demand a second and independent separation step in order to seperately analyse the multiplicity of compounds at lowest (ppb) concentration levels.
To overcome the limitation of IMS with regard to separation efficiency G.A.S. equips almost all of its IMS systems with gas chromatographic (GC) columns. The volatile compounds of samples under investigation are pre-separated in time by a GC column. The discrete compounds elute directly into the IMS ionization chamber, so that analyte and/or ion interactions and a competition of analytes on the reactand ions can be avoided. By that the GC-IMS set-up enables a twofold separation of analyte mixtures and the detection by the IMS‘ electrometer. The separation of compounds based on their retention indices (RI) both offers information for identification (using the NIST Kovats RI-data base) and suppresses undesired compound interactions during ionization and detection in the IMS. This configuration enhances the selectivity of the detector towards individual compounds.
Since the IMS measurements are extremely fast (up to 21ms/ spectrum) a continuous and high-resolution recording of analyte signals is provided. Shown figure sketches the GC-IMS sample flow and detection leading to a 3D-dataset of GC- and IMS separation, same as the corresponding intensity which are processed using G.A.S. VOCal and additional software tools.
In order to optimize the analytical performance if its Time-OF-Flight IMS further G.A.S. recently developed a special ionisation architecture that enhances the compound feed time resolution shaping a downsized, ‘virtual’ void volume controlled by a more effective sample- and drift gas flow inside the ionization area. This FOCUS-IMS® set-up (patent pending) leads to a correlation between Signal peak shape and Electronic Pressure Controller (EPC) applied flow rates. Peak symmetry and Full Width at Half Maximum (FWHM) become comparable to state-of-the-art Mass Spectrometers.
The ratio of carrier- and drift gas controls the size and dwell time of the sample in the ionization sphere and by that becomes a tuneable parameter by the operator to optimize sensitivity and flush out characteristics acc. to the application requirements. As a consequence, an elevated drift gas flow lowers the sensitivity throughout the whole concentration range and leads to the effect that the linear range of the FOCUS-IMS® can be increased significantly compared to classical IMS designs (see Technical Note).
GC-IMS Data Analysis Software - VOCal
G.A.S. offers a comprehensive line-up of software tools supporting automated data acquisition as well as data visualization, evaluation, calibration, quantification and characterization.
 Deutsch
Deutsch English
English Chinese
Chinese


