IMS Working Principle
Ion Mobility Spectrometry (IMS) is an analytical technology to separately
detect gaseous compounds in a mixture of analytes. The separation is based on
the specific drift times, that ionized compounds need to pass a fixed
distance (drift tube) in a defined electric field.
Compared to other techniques e.g. TOF-MS, ions travel at atmospheric
pressure versus a flow of inert drift gas. The drift time of each
substance is determined by its ion‘s mass and geometric structure, as slowing
collisions with the drift gas molecules are more frequent for sterically
demanding structures. Therefore IMS can even differentiate isobaric molecules.
For detection, the resulting ion current is measured by an electrometer as a
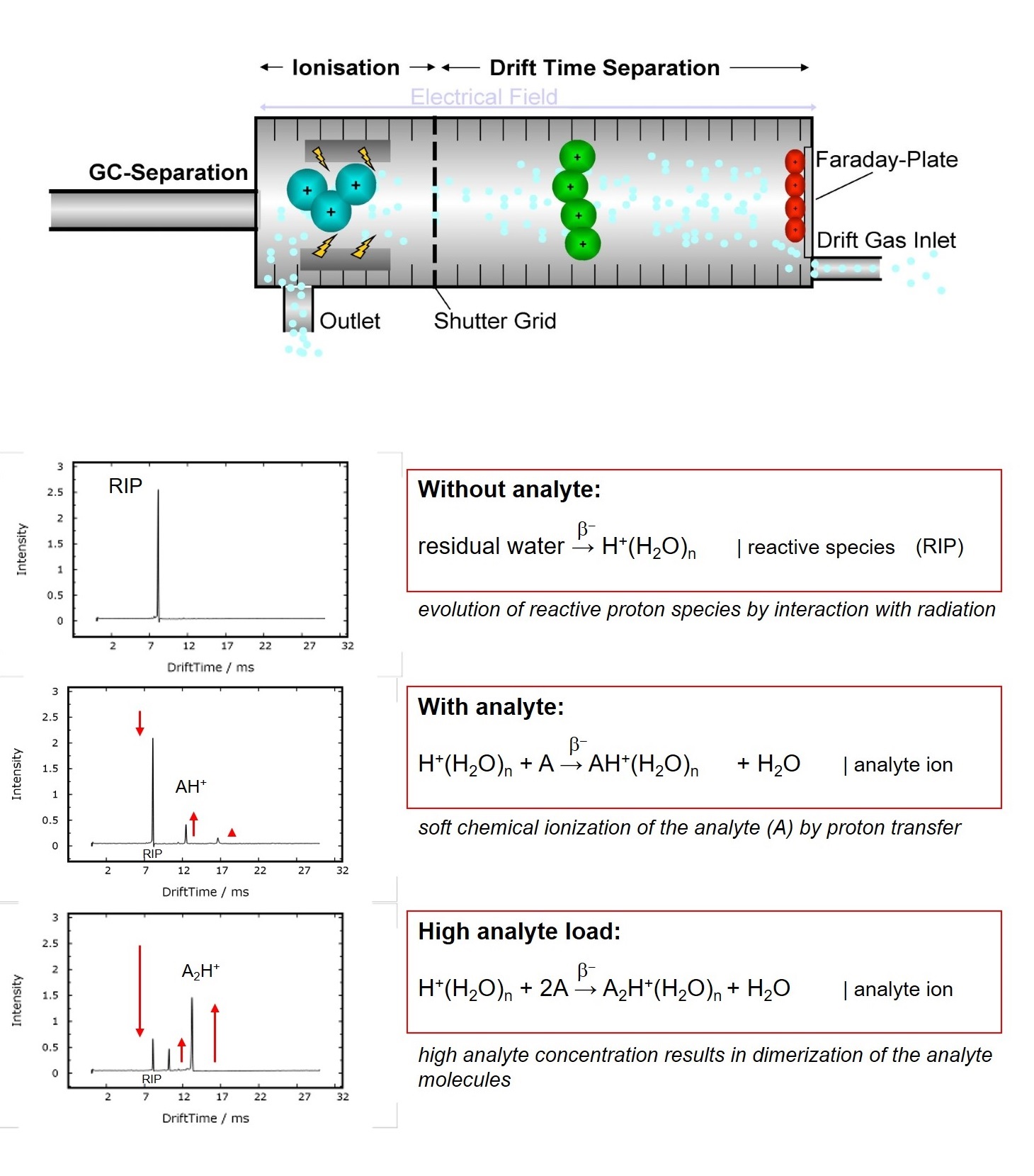
function of time. Atmospheric Ionization of molecules can be obtained by several
techniques. G.A.S. uses soft chemical-ionization initiated by a low-radiation tritium (H3) source (below
excemption limits of IAEA resp. EURATOM).
In a first step rectand ions are generated by a cascade of reactions following the collision of a fast electron emitted from the β-radiator with the drift gas atmosphere[1]. As a consequence the so-called Reaction Ion Peak (RIP) representing the total of all ions available is formed. In nitrogen and air, resp., the reactand ions can be descibed as H+(H2O)n and O2-(H2O)n. Chemical ionization of analytes by reactand ions then result in the formation of specific analyte ions, when the affinity of the analyte towards the reactand ion is higher when compared to water. The proton affinity of water is 691kJ/mol, so all molecules with a higher proton affinity will be ionized by proton transfer, which is typically given for all heteroatom-organic compounds.
[1] Eiceman, G. and Karpas, Z., Ion Mobility Spectrometry, ISBN 0-88493-2247-2
 Deutsch
Deutsch English
English Chinese
Chinese


